Research has demonstrated that humans need a large number of trace minerals to maintain health. Of these, zinc is the second most abundant trace mineral in the body after iron. In fact, zinc is present as a co-factor in more enzymes than all the other trace minerals combined. Just as the selenium ion is necessary for the function of the selenoproteins (here), the zinc ion is necessary for the zinc metalloproteins. Zinc ions are needed by the metalloproteins because they either act directly as a catalysts, or their charge maintains the structure and shape of the enzymes. Without this zinc, the enzyme losses its catalytic effects or denatures into a structure that is unable to recognise its substrate. Absence of zinc from the metalloproteins results in metabolic disorder and disease.
Zinc metabolism in the body (figure 1) begins when zinc is ingested in the diet. Zinc absorption is normally around 20 %. Binding zinc to organic compounds such as citric acid and picolinic acid appear to increase the absorption of zinc. Calcium (here), oxylate, phytate, dietary fibre and divalent cations can inhibit zinc absorption. Once in the blood zinc is transported bound to albumin where it is passed to tissues by an amino acid carrier. Zinc is stored in cells on a protein called metallothionein or incorporated into metalloproteins. When zinc stores become low, metallothionein releases stored zinc and the less essential zinc metalloproteins are catabolised to release their zinc ions. Zinc is actively secreted from the salivary glands, intestinal mucosa and pancreas and reabsorbed or lost in the faeces. Some zinc is also lost through sweating and exfoliation of skin.
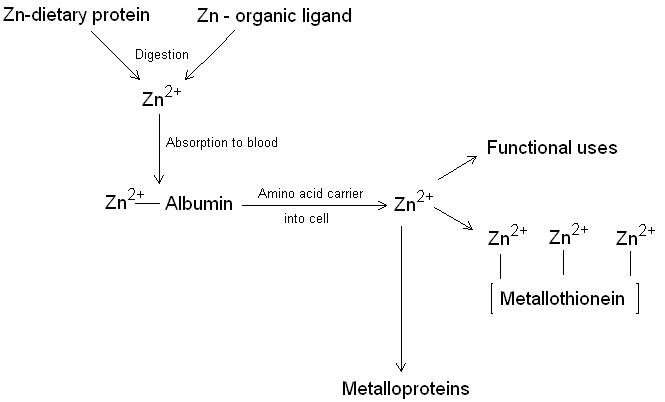 Figure 1. The metabolism of zinc showing the incorporation of Zn2+ into the zinc metalloproteins and metallothionein.
Figure 1. The metabolism of zinc showing the incorporation of Zn2+ into the zinc metalloproteins and metallothionein.
Zinc plays an important role in the body as an antioxidant. Zinc is a co-factor for a group of metalloprotein enzymes called superoxide dismutase. Two atoms of zinc, along with two atoms of copper, are necessary for the function of this enzyme which is located in the cytosol of the cell. Another form of superoxide dismutase that requires manganese as a co-factor is located in the mitochondria. Superoxide dismutase catalyses the removal of the superoxide radical (O2-) to form hydrogen peroxide and oxygen (figure 2). Deficiency of either copper or zinc results in an impairment of the cytosolic superoxide dismutase enzyme. Metallothionein is also a reducing agent and may function as an antioxidant within cells. Zinc may prevent oxidation of membranes by occupying sites in place of other pro-oxidant metals.
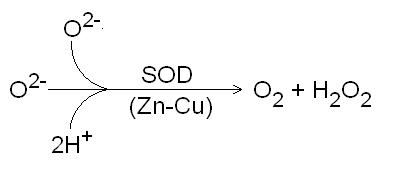 Figure 2. Superoxide dismutase (SOD) catalysing the conversion of the superoxide radical to hydrogen peroxide and oxygen.
Figure 2. Superoxide dismutase (SOD) catalysing the conversion of the superoxide radical to hydrogen peroxide and oxygen.
Carbonic anhydrase is a zinc metalloprotein that is found in the erythrocytes and renal tubules. Carbonic anhydrase plays an important role in promoting carbon dioxide exchange, maintaining acid-base balance and promoting bicarbonate secretion; by catalyzing the reversible conversion of carbon dioxide to bicarbonate. Carbonic anhydrase has a very high affinity for the zinc ion and as such does not readily give up its zinc during time of zinc deficiency. In contrast, zinc deficiency will cause levels of the enzyme alkaline phosphatase to drop as the zinc ions are catabolised for other functions. Alkaline phosphatase contains four zinc ions per enzyme, with two enzymes needed to maintain the shape of the enzyme and two needed for catalysis. Alkaline phosphatase removes phosphate groups from molecules in a dephosphorylation reaction.
Other zinc metalloproteins include alcohol dehydrogenase, carboxypeptidase, aminopepetidase, phospholipase C and δ-aminolevulinic dehydratase. Like alkaline phosphatase, alcohol dehydrogenase also contains four molecules of zinc, with two required for conformation of the protein and two required for catalytic activity. Alcohol dehydrogenase catalyses the conversion of alcohols to aldehydes such as retinol to retinal, the form of vitamin A necessary for vision. Aminopeptidase and carboxypeptidase are digestive enzyme that are involved in protein digestion in the gut. Aminopeptidase and carboxypeptidase cleave amino acids from the amino-terminus and carboxy-terminus of dietary proteins respectively. Phospholipase C cleaves the phosphodiester bond from phospholipids to release PO3 which is important in cell signal transduction. δ-aminolevulinic dehydratase is an enzyme that contains eight zinc ions in each of its eight subunits and is necessary for the formation of haem in haemoglobin.
In addition to the metalloproteins, zinc has many other functions in the body. Zinc is incorporated into insulin, and deficiency of zinc results in poor insulin response to carbohydrates. Zinc defocoency has also been shown to affect basal metabolic rate (here) by reducing thyroid hormone levels. Zinc may also stabilise cell membranes by maintaining thiol groups in a reduced state, allow binding of steroid hormones to DNA (as ‘zinc fingers’), and is required for taste acuity. When zinc intake in the diet is insufficient to meet metabolic demands, some of the zinc metalloproteins are catabolised to yield their zinc ions. Therefore, maintaining adequate intake is essential if the zinc metalloproteins are to be protected from destruction. Obtaining zinc from food is difficult, and so supplementation with an absorbable form of zinc is the best way to ensure zinc sufficiency.
RdB
