Many of the agricultural soils of Northern Europe and North America have low levels of selenium. Intensive agricultural practices and over farming have depleted the soil further of their selenium content, and a growing body of research is confirming that our foods are deficient in this important trace mineral. If you have been reading my previous articles, you will already be aware of the importance of selenium in the diet. Selenium is needed for the normal functioning of a number of metabolic pathways, because it is incorporated into a group of enzymes called the selenoproteins. Like many enzymes, selenoproteins are able to catalyse reactions in metabolic pathways because they contain metal atoms within their structures which maintains their biological activity. When the selenium intake is low and we become deficient, the selenoproteins cannot function and diseases develop.
Selenium metabolism (figure 1) begins when selenium compounds in the diet are absorbed. Inorganic selenite (SeO32–) is absorbed at roughly 40 to 70 %, a slightly high rate that for inorganic selenate (SeO42–). Better absorption is seen with the organic selenomethionine (available as selenium yeast) and selenocysteine which improves absorption to 50 to 80 %. Antioxidants such as vitamin C, vitamin E and reduced glutathione in high concentrations in the intestinal lumen tend to improve absorption of selenium. The ingested selenocompounds are metabolised to selenoglutathione, and ultimately generate selenide. The next step is the synthesis of selenophosphate, which can be metabolised and its selenium subsequently attached to transfer RNA for synthesis of selenocysteine. Selenocysteine is then incorporated into a number of important selenoproteins. If selenide is not converted to selnophosphate then it is sequentially methylated and excreted through urine or exhaled.
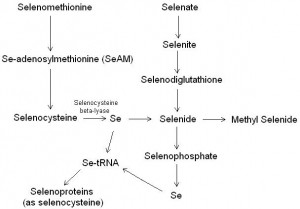 Figure 1. The metabolism of selenium. Inorganic selenate or selenite (in the diet) are metabolised to selenide. Selenide can be methylated and excreted Alternatively selenide can be metabolised to selenophosphate, and then its selenium can be incorporated into selenoproteins via transfer RNA (tRNA). Organic selonomethionine (from yeast) is metabolised first to selenocysteine. The enzyme selenocysteine beta-lyase can then cleave elemental selenium, which can then be inporporated into selenide or the selenoproteins via transfer RNA.
Figure 1. The metabolism of selenium. Inorganic selenate or selenite (in the diet) are metabolised to selenide. Selenide can be methylated and excreted Alternatively selenide can be metabolised to selenophosphate, and then its selenium can be incorporated into selenoproteins via transfer RNA (tRNA). Organic selonomethionine (from yeast) is metabolised first to selenocysteine. The enzyme selenocysteine beta-lyase can then cleave elemental selenium, which can then be inporporated into selenide or the selenoproteins via transfer RNA.
Selenocysteine is ionised and as such makes a very efficient biological catalyst. Selenocysteine is incorporated into a number of selenoproteins, the most widely known being glutathione peroxidase (GPx). Glutathione peroxidase catalyses the reduction of hydrogen peroxide (H2O2) in the cytosol and mitochondria of cells as well as plasma. The cellular tripeptide glutathione serves as a substrate for glutathione peroxidase during these reactions. In the process glutathione becomes oxidised as two molecules give up their hydrogen atoms from their sulfadryl groups (figure 2). The two sulfhydryl groups then join to form a disulfide bond. Glutathione peroxidase can also catalyse the reduction of lipid peroxides. Because it contains four molecules of selenium, glutathione peroxidase may also be a storage vehicle for selenium. Five glutathione reductase enzyme are known, but only the first four are selenium dependent.
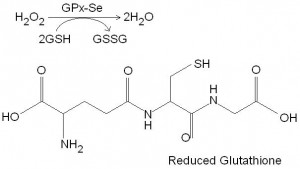 Figure 2. The molecular structure of reduced glutathione (GSH) (bottom). Notice the sulfydryl group (-SH). Also shown is the catalysis of hydrogen peroxide to water by glutathione peroxidase, a selenoprotein with four atoms of selenium (top). This reaction requires two molecules of reduced glutathione as a substrate to donate two atoms of hydrogen (from the -SH groups). Two molecules of reduced glutathione then join to form the oxidised form (GSSG).
Figure 2. The molecular structure of reduced glutathione (GSH) (bottom). Notice the sulfydryl group (-SH). Also shown is the catalysis of hydrogen peroxide to water by glutathione peroxidase, a selenoprotein with four atoms of selenium (top). This reaction requires two molecules of reduced glutathione as a substrate to donate two atoms of hydrogen (from the -SH groups). Two molecules of reduced glutathione then join to form the oxidised form (GSSG).
Another important class of selenoproteins are the iodothyronine deiodinases, which contain a single selenium atom. There are three types of iodothyronine deiodinases, which are found in varying concentrations in the endoplasmic reticulum of liver, kidney and muscle cells. Iodothyronine deiodinases catalyse the deiodination of tetra-iodothyronine (thyroxine, T4) to tri-iodothyronine (T3) and reverse tri-iodothyronine (rT3) (figure 3). This reaction is important for the production of the main active thyroid hormone (T3) and is required for normal growth and metabolic regulation. The ratio of T4 to T3 has been identified as an important marker of thyroid function, and the ratio of T4 to T3 tends to decrease with age. Research has shown that supplementing with selenium can reverse this reduction, suggesting that selenium deficiency causes metabolic slow down as we age.
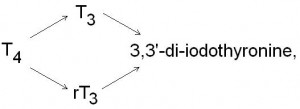 Figure 3. The conversion of tetra-iodothyronine (T4) to tri-iodothyronine (T3) and reverse tri-iodothyronine (rT3). Tri-iodothyronine and reverse tri-iodothyronine are then metabolised to 3’3-di-iodothyronine.
Figure 3. The conversion of tetra-iodothyronine (T4) to tri-iodothyronine (T3) and reverse tri-iodothyronine (rT3). Tri-iodothyronine and reverse tri-iodothyronine are then metabolised to 3’3-di-iodothyronine.
Other selenoproteins include thioredoxin reductase, selenoprotein P and selenoprotein W, although less is known about these proteins. Thioredoxin reductase is an enzyme known to catalyse the reduction of thioredoxin using NAD(P)H as a hydrogen donor. Thioredoxins are a group of cellular proteins that have important cellular effects that may include antioxidant functions. For example, they are able to reduce cellular thiol containing compounds such as lipoic acid (LA) and glutathione (GSSG) to dihydrolipoic acid (DHLA) and reduced glutathione (GSH) respectively. In addition, thioredoxins are also able to recycle vitamin C. Selenoprotein P contains 10 atoms of selenium per molecule and may therefore serve as a transporter of selenium in plasma, although its distribution in tissue may suggest other functions as well. Selenoprotein W plays a role in muscle metabolism although much less is known about its actual function.
Tissue levels of selenium are influenced by selenium intakes. Because there is a wide variation in soil concentration of selenium, supplementation is essential to be assured an adequate intake. Many of the studies looking at selenium and health have used the organic yeast forms of selenium, and this appears to be effectively absorbed and incorporated into to tissues. Inorganic selenite and selenate are not so well absorbed and are probably best avoided unless there is no other choice. Clinical signs of acute deficiency are very rare for selenium, and as a result little attention is paid by the mainstream medical establishment to selenium levels in diets. However, a growing body of research suggests that selenium is pivotal in out long term protection from cancer, heart disease and metabolic disorders that take decades to develop.
RdB
