Stress causes adaptation and allows beneficial changes to occur in organisms. For example, exercise is a form of stress that forces physiological adaptations to improve physical performance. However, following application of a stressor, time must be allowed for adaptation. During this time, energy is used to remodel cellular and tissue components, subsequently providing a benefit. If this period of remodelling is cut short by further application of a stressor, the adaptation phase is not completed and disruption of cellular systems occurs. If a stressor is repeatedly applied beyond the ability of the organism to recover, a state of semi-permanent dysfunction, characterised by endocrine and nervous system imbalance, is initiated. If allowed to continue, such a situation causes ill-health and ultimately death. This is the basic model of stress first describes by Hans Selye and is named the general adaptation syndrome.
Stress can come in many forms and the perception of the individual can often dictate what does and does not constitute a stressor. However, a scientific definition of stress could be described as deviation of normal biological parameters from their homeostatic norm. Deprivation of sleep is a well know stressor and its accumulative effect form the basis of torture for this reason. Although the purpose of sleep is not fully understood it is generally accepted to have restorative functions. The brain is particular vulnerable to sleep deprivation and deleterious cognitive changes are observable even with minimal deprivation. The changes that occur following sleep deprivation have been studied in detail and can range from poor judgement, confusion and irritability to hallucination and death. It is thought that mild sleep deprivation is common and so scientists are interested in its chronic effects.
From a nutritional perspective, recent evidence suggests that sleep deprivation may disrupt the appetite systems that control food intake. Energy balance is controlled by a hormonal system that relates information regarding the energy stores, blood sugar and the content of the gut to centres in the hypothalamus of the brain for processing (figure 1). Insulin and ghrelin are hormones that are though to have a strong influence on this system, and in turn these stimulate release of either orexigenic or anorexigenic peptides increase or decrease satiety. Some evidence suggests that sleep deprivation causes an imbalance in is system such that plasma concentrations of the satiety hormone leptin fall, whereas those of the appetite stimulating hormone ghrelin rise. The response to excessive sleep deprivation may therefore be modulation of diet and a possible increase in energy consumption experienced in the subsequent period. 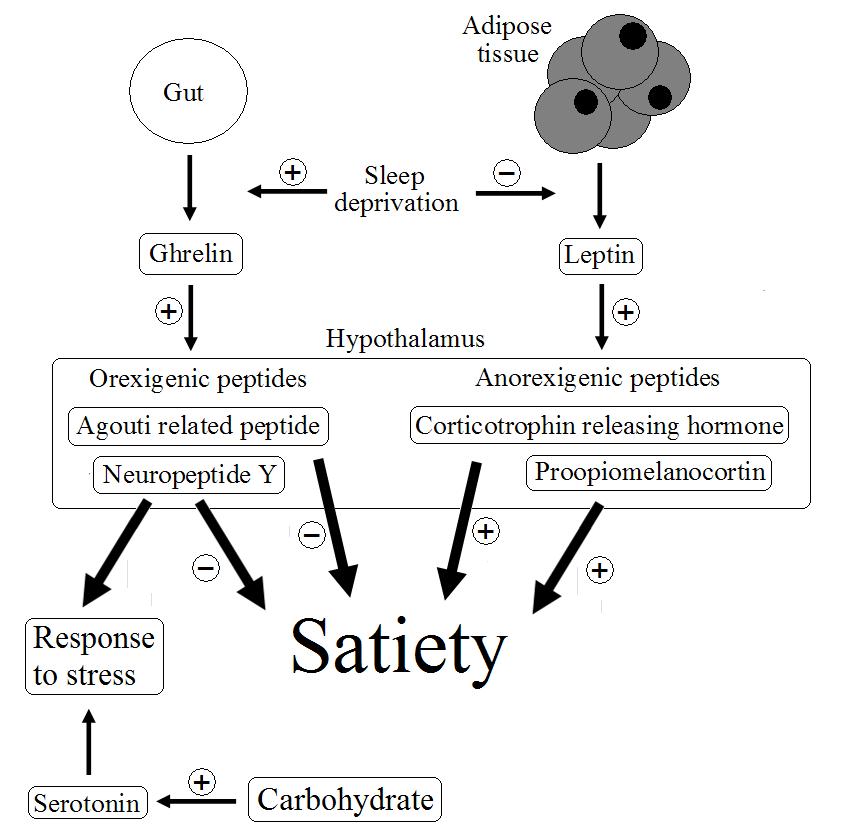 Figure 1. Possible mechanisms in the regulation of appetite, relating to the response to stress.
Figure 1. Possible mechanisms in the regulation of appetite, relating to the response to stress.
Researchers1 have investigated the effects of sleep deprivation on neuronal activation in response to food stimuli. In a cross-over design study, 30 normal weight individuals were allowed either 4 hours or 9 hours sleep per night, to represent mild sleep deprivation or normal sleep patterns, with each phase lasting 6 days. The authors then used magnetic resonance imaging (MRI) to build a picture of the neuronal activity in the brain that occurred in response to a variety of food images. Following sleep deprivation the MRI scan showed increased neuronal activity compared to baseline. Interestingly the areas of the brain associated with reward, the putamen, nucleus accumbens, thalamus, insula and prefrontal cortex were particularly stimulated following sleep deprivation. The authors concluded that sleep deprivation has a significant effect on the brain areas that are associated with reward suggesting that this may lead to a propensity to overeat.
These results are difficult to interpret in isolation because of the complexity of the brain, but it appears that sleep deprivation in some way sensitises particular areas of the brain to the stimuli from food. The same authors have previous published research to show that sleep deprivation increases food intake (here), but these results provide the first evidence that sensitisation of particular areas of the brain might be the mechanism by which this occurs. Both serotonin and neuropeptide Y are strongly implicated in the response of the brain to stress. Neuropeptide Y is released in response to stimulation by ghrelin and serotonin levels in the brain increase in response to carbohydrate foods and insulin release. Appetite might therefore be stimulated following stress such as sleep deprivation in order to provide adequate levels of these chemicals. Sensitising certain areas of the brain may improve this response.
RdB
