Flavonoids represent a group of over 4000 compounds that contribute towards the colours in many plants. Consumption of fruits and vegetables is known from epidemiology to provide projection against certain diseases and flavonoids have been extensively researched in association with this protection. Flavonoids have been shown to be bioavailable in humans although the exact mechanisms of action are not fully understood. Because flavonoids represent a diverse group of chemicals, it would be expected that biological interactions would vary between compounds based on their structures. Indeed, this is exactly what the literature demonstrates, an overlapping but distinct structure function relationship for the various flavonoids, with ring structure and β-ring hydroxylation pattern playing a particular important role in determining biological function. Polymerisation may also affect the biological properties by altering their absorption.
One group of flavonoids, the anthocyanins, have been extensively researched in relation to their ability to prevent disease. Anthocyanins are glycosylated flavonoids in plants that are though to be produced as a reaction to stressors such as UV light, cold, and drought. The characteristic carbon structure of the anthocyanins is shown in figure 1. The conjugated double bonds that carry a positive charge on the heterocyclic oxygen ring, results in a chromophoric molecule with maximum absorption of white light between 456nm and 550nm and UV light between 270nm and 280nm and results in intense red and orange colour in acidic conditions and violet and blue colours under alkaline conditions. Over 90% of the 635 identified anthocyanins in nature belong to a small group of well characterised flavonoid glycosides that include cyanidin, delphinidin, malvidin, pelargonidin, peonidin and petunidin.
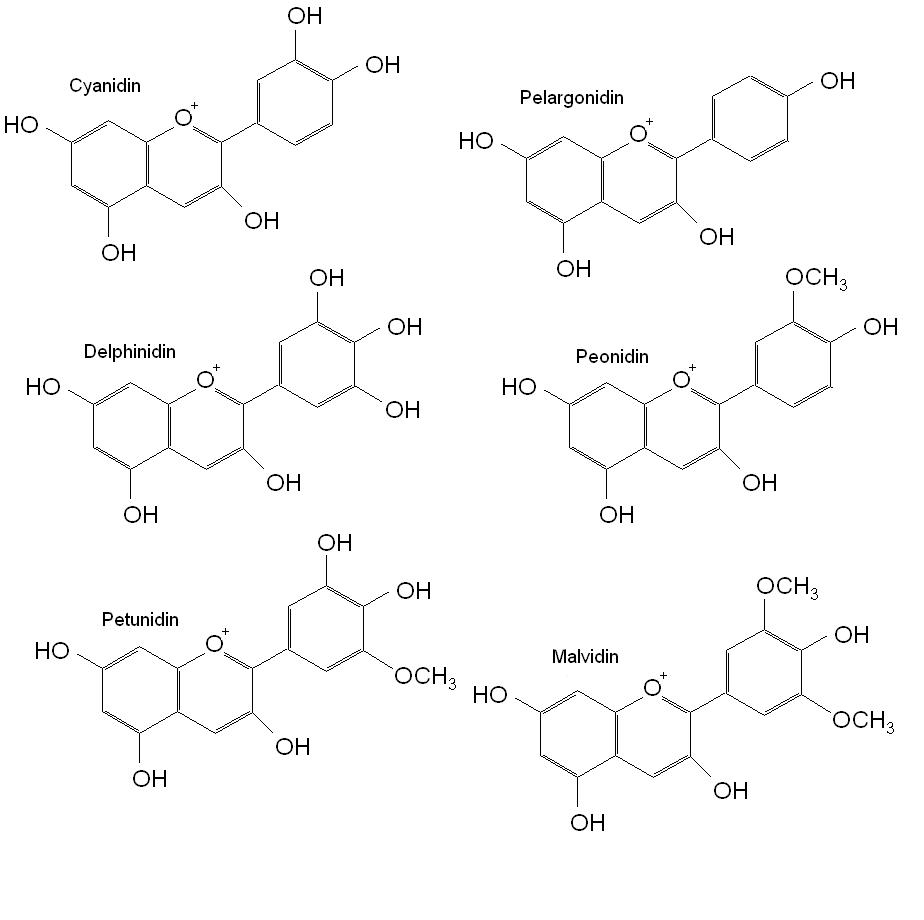 Figure 1. Anthocyanidin structure. Anthocyanins have a similar structure, but with addition of a glycosidic bond linking a sugar moiety (the glycoside form).
Figure 1. Anthocyanidin structure. Anthocyanins have a similar structure, but with addition of a glycosidic bond linking a sugar moiety (the glycoside form).
Anthocyanins are bioavailable and their absorption involves removal of the glycoside sugar moiety (via glycosidase) to form their corresponding anthocyanidin aglycone, which then diffuses across the intestinal mucosa (here). The uptake of anthocyanidins (7.5% of blackberry anthocyanins absorbed in rats), as with most flavonoids, appears to be greater than can be accounted for by their urinary and plasma concentrations. This suggest that there is an uptake of the anthocyanins into the enterocytes of the gut, but that they are not transported basolaterally to the circulation. This may reflect the fact that the enterocytes are highly metabolically active and the anthocyanins are extensively metabolised by phase II conjugation enzyme, after which they are then effluxed back to the gut lumen. Altering the sugar moiety from a glucose molecule to a galactose or arabinose molecule in anthocyanins may decrease the absorption rate, at least for cyanidin or peonidin.
The absorption of anthocyanins may be influenced by the food matrix they are contained within, as urinary anthocyanin recovery from strawberries (≈2%) is lower than that from cranberry juice (≈5%). In addition, genetic polymorphism for the main metabolising enzymes UDP glucuronsyl transferase, glutathione S-transferase and catechol-O-methyltransferase may alter the rate of phase II metabolism in enterocytes. Increased metabolism within enterocytes may decrease absorption by increasing apical efflux of conjugated anthocyanins back to the gut lumen. The extensive phase II metabolism and a second round of further metabolism in the liver is reflected by the presence of a mixture of glucuronidated, sulphated and methylated metabolites in plasma 1 to 3 hour post consumption. Anthocyanins can still be detected in the urine for up to a day after consumption. With incrementally increasing methylation causing probably alterations in biological activity during this time.
Absorption of anthocyanins may occur via the colon following metabolism by gut microflora as many bacteria in the human intestine have the ability to metabolise anthocyanins, anthocyanidins and anthocyanin metabolites. This metabolism is not fully understood and is complex, but likely results in the formation of simpler structured phenolic compounds that are bioavailable in humans. These products may include syringic acid, vanillic acid, phloroglucinol aldehyde, gallic acid and phloroglucinol acid. In addition, gut bacteria possess the enzymes necessary to deglycosylate flavonoids, and so it is possible that anthocyanins not absorbed in the small intestine may pass to the colon where bacteria convert them to anthocyanidins which are them absorbed due to their increased lipophilicity. Simpler phenolic compounds may retain or even increase the antioxidant activity of their parent compounds and so extensive colonic metabolism may not be detrimental.
RdB
