Flavonoids give some plants their distinctive colours. Flavonoid structure may affect the biological activity of flavonoids.
Flavonoids represent a group of antioxidants that may show bioactive properties in humans and animals. Because they have been extensively researched and found to show possible health benefits in humans, both the mainstream media and health industry have widely reported flavonoid research. Flavonoids are present in many foods of plant origin, and common foods such as apples, chocolate, and onions and beverages such as tea and wine are rich sources. Certain plants and spices containing flavonoids have been used for centuries in traditional Eastern medicine and a variety of flavonoids are being sold in dietary supplements. However, Western medicine has not yet used flavonoids therapeutically, despite voluminous literature and an exceptional safety record.
Epidemiological studies suggest that dietary flavonoids may play a part in protecting humans from chronic diseases. In vitro, flavonoids have been shown to induce responses consistent with the protection afforded by diets rich in fruits and vegetables. In recent years, flavonoids have attracted increasing interest due to their various beneficial pharmacological effects including anti-inflammatory, anti-cancer and anti-atherogenic properties. Large amounts of in vitro data have been collated concerning flavonoids, although less data are available from human and animal studies. Cell culture studies have increased the understanding of flavonoid structure, metabolism and absorption in humans in recent years, but much work is still needed in order to fully understand flavonoid absorption, particularly from the point of view of individual flavonoid conjugates following extensive enterocyte metabolism.
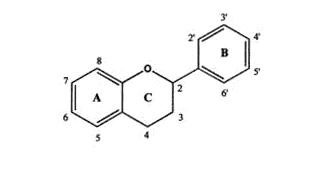
Flavonoids belong to a group of natural compounds with variable low molecular weight polyphenolic structures found in material of plant origin. The flavonoid structure is based on a flavan nucleus which consists of three phenolic rings referred to as the A, B and C rings (figure 1.). The benzene ring A is condensed with the six member C ring, which in the 2-position carries the B ring as a substitute. The basic flavonoid structure allows a large number of substitutions in the three rings; variable numbers of hydroxyls may be further substituted with sugars, methyl groups, sulphates and glucuronides, thus producing an extremely diverse range of derivatives. More than 4000 different naturally occurring flavonoids have so far been identified, many of which are responsible for the vivid colours of leaves, fruits and flowers.
The classification of flavonoids is based on their structure. Variations in the heterocyclic C ring give rise to the five major classes: flavan-3-ols, flavones, flavanones, flavonols and anthocyanidins. Isoflavones and chalcones are not, according to strict criteria, classified as flavonoids but they share similarities in physico-chemical properties and are often included when flavonoids are referred to (figure 2). The chemical nature of flavonoids is closely related to their structure. Factors that can influence their chemical nature include structural class, degree of hydroxylation, degree of polymerisation, as well as substitution and conjugation. The pattern of substitution can be very complex, can modify the hydrophobicity of the molecule and its biological properties, and markedly increase the molecular weight of the flavonoid.
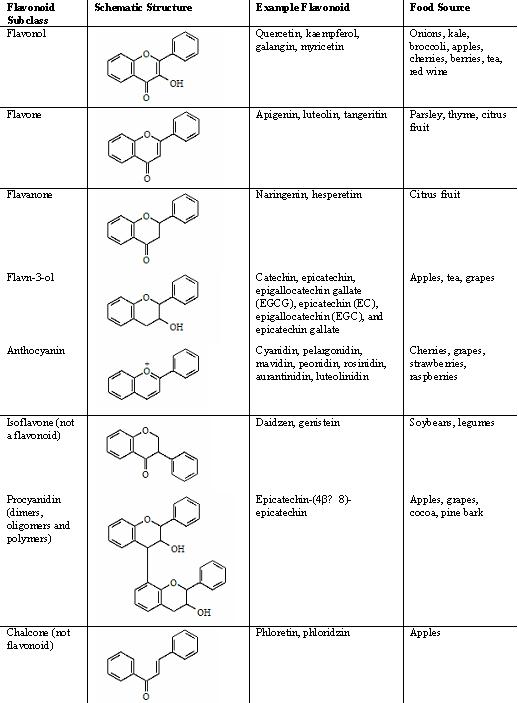
Figure 2. Flavonoid structure. The main classes of flavonoids; flavonol, flavan-3-ol, flavone, flavanone and anthocyanin. Also shown are chalcones and isoflavones, not true flavonoids, but often grouped together. Also shown is the flavonoid polymer, proanthocyanidin, made up of flavan-3-ol units. Adapted from Hollman and Katin, 1997; Middleton et al, 2000; Cuyckens and Claeys, 2004.
Flavonoids can function as scavengers of free radicals by rapid donation of hydrogen atoms to radicals and their antioxidant nature has been extensively reviewed. Many studies have investigated this antioxidant activity of flavonoids, and many attempts have been made to establish the relationship between flavonoid structure and antioxidant activity. The molecular structure and substitution pattern of the hydroxyl groups is in large part responsible for the antioxidant ability of the flavonoid molecules. In particular, the availability of the phenolic hydrogens and the ability to allow stabilisation of the resultant phenoxyl radical via hydrogen bonding or expanded electron delocalisation. A number of structure-activity relationship studies of flavonoids have highlighted the importance of the location and number of phenolic hydroxyl groups present for their antioxidant activity.
The structural requirement considered to be most important for effective radical scavenging by flavonoids is the presence of a 3′, 4′-dihydroxy (catechol structure) in the B ring (e.g. quercetin, luteolin, catechin). Other structural features are considered beneficial for antioxidant activity including the 3-OH moiety of the C ring (e.g. flavonols and the flavn-3-ols), a C2-C3 double bond conjugated with a 4-keto group (e.g. flavonols and flavones) as well as the presence of both 5-OH and 3-OH groups in combination with a 4-keto group (e.g. flavonols). Saturation of the 2, 3-double bond is believed to cause a loss of antioxidant potential (e.g. flavanones). In the absence of the O-dihydroxy structure in the B ring (e.g. 5-OH and 3-OH groups), hydroxyl constituents in a catechol structure on the A ring are able to compensate and become a larger determinant of flavonoid antioxidant potential (e.g. naringenin). Linkage type C4-C6 (e.g. procyanidin B5) or C4-C8 (e.g. epicatechin-(4β→8)-epicatechin) have also been shown to exert a substantial effect upon antioxidant activity of procyanidins.
Based on the structural requirements for effective free radical scavenging activity it would appear that amongst the flavonoids, the flavonols should show excellent promise as antioxidants, and they do. Catechin and luteolin have also been demonstrated to possess good antioxidant ability. Research to date has focussed mainly on quercetin and catechin, showing benefits to both dietary supplements and foods such as tea and onions. As well as its good antioxidant effects, quercetin has been shown to have good anti-inflammatory effects due to the presence of the double bond at the C2-C3 position and the 3′ and 4′ hydroxylations on the B ring. The antioxidant and anti-inflammatory effects of quercetin make it a useful nutrient in the fight against diseases characterised by these processes, particularly arthritis and heart disease. However, the diverse effects of other flavonoids may also be useful to health, and so incorporation of a wide range of flavonoids from a varied diet is a very sensible step in the journey for optimum nutrition.