Conjugated linoleic acid (CLA), also called alpha-rumenic acid, is a collective term that refers to a mixture of isomers of linoleic acid [18:2(n-6)]. The two double bonds in CLA are separated by a single bond (conjugated) and can be in the cis or trans configuration. The combinations of cis and trans bonds in positions 9 and 11 or 10 and 12 gives rise to the different geometric isomers (figure 1). Meat from ruminants (e.g. mutton and beef) is the major source of CLA in the human diet, because ruminants synthesise CLA in their stomachs from cud. Milk, cheese and other dairy products are also good sources. Evidence suggests that the trans-10, cis-12 isomer can prevent the development of obesity, at least in animal models (here). Even in small amounts (≤ 1 % of diet) CLA appears to show inhibitory effects on carcinogenesis.
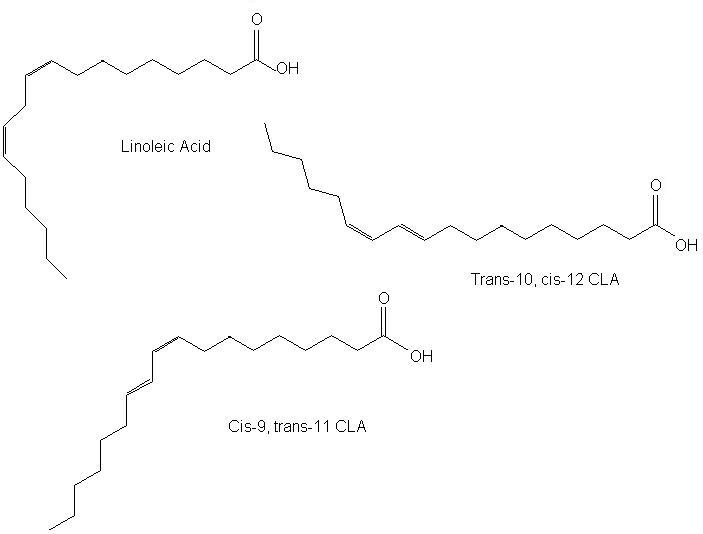 Figure 1. The structure of linoleic acid and two conjugated isomers.
Figure 1. The structure of linoleic acid and two conjugated isomers.
Both the free fatty acid and triacylglycerol forms of CLA have shown to have an inhibitory effects on carcinogenesis. In addition, experiments on rats have demonstrated that this inhibitory effect of CLA is present irrespective of the other fat consumed in the diet. This same research also showed that CLA does not appear to display interference with the metabolism of the essential fatty acid linoleic acid [18:2(n-6)]. Rat studies have shown that increasing the amount of CLA in the diet above 1 % does not increase the protective effect against carcinogenesis. This may indicate that there is a rate limiting step in the metabolism of CLA into some active product, that is essential for the protective effects on carcinogenesis. Research suggests therefore that CLA is an effective anticancer compound even at very low concentrations in the diet.
RdB
