The catecholamines are a group of neurotransmitters that comprise of the chemicals dopamine, adrenaline and noradrenaline. They are synthesised and released by nerves of the adrenergic pathways. Adrenergic pathways activate the sympathetic branch of the autonomic nervous system. Therefore catecholamines are important in the arousal of emotional and motivational behaviour. By regulation of the hypothalamus the catecholamines also have an impact on many bodily systems through the release of hormones. Because of these effects, catecholamines can be an important determinant of mental and physical performance. Substances such as cocaine, amphetamine and ephedrine all have a history of abuse by athletes and all act to synthetically increase certain catecholamine neurotransmitters thereby altering the physical, mental and psychological parameters of the human body. Decreasing catecholamine production in the brain as a consequence of stress and can result in poor physical and mental performance.
Tyrosine hydroxylase is the rate limiting step in the synthesis of all catecholamines from the amino acid phenylalanine (figure 1). Regulation of tyrosine hydroxylase activity therefore controls catecholamine production. A number of factors are known to regulate the activity of tyrosine hydroxylase and this regulation centres of the control of its iron cofactor. The unphosphorylated enzyme with ferrous iron is fully active because the tetrahydrobiopterin (BH4) coenzyme maintains the ferrous iron in its reduced state. Displacement of the tetrahydrobiopterin coenzyme causes the iron to convert to its ferric form, thus decreasing the activity of the enzyme. This can occur through increased formation of catecholamine which bind to the enzyme and displace the tetrahydrobiopterin. Thus increasing production of the catecholamines causes feedback inhibition of tyrosine hydroxylase activity, and the catecholamines therefore regulate their own production. This illustrates the importance of iron in the central function of the tyrosine hydroxylase enzyme.
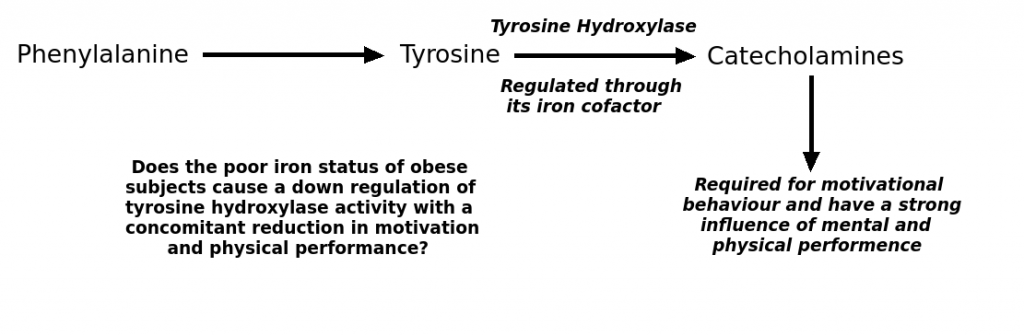
The synthesis of catecholamines from tyrosine requires the enzyme tyrosine hydroxylase. Iron is a co-factor for tyrosine hydroxylase.
Because iron is central to the activity of tyrosine hydroxylase, low iron status could reduce the activity of the enzyme. Indeed, experiments on rodents show that iron deficiency lowers the activity of tyrosine hydroxylase in liver tissue. Also human studies have been performed and show a role for iron deficiency in down regulating the rate of tyrosine hydroxylase activity. For example, in one study researchers used a phenylalanine load test using radiolabelled L-phenylalanine [L-(2H5)phenylalanine] to investigates the effects of iron deficient anaemia on tyrosine hydroxylase activity1. Fasting phenylalanine to tyrosine ratios in plasma were determined and then radiolabeled phenylalanine was introduced intravenously. Measurements of radiolabeled tyrosine production then allowed estimation of tyrosine hydroxylase activity. The results showed that iron deficient anaemia produces tyrosine hydroxylase activity part way between normal healthy subjects and those with a complete genetic deficiency of tyrosine hydroxylase.
Obesity is characterised by metabolic dysfunctions. One such metabolic dysfunction has been shown to be in iron homeostasis (here). Obese individuals have an increased risk of iron deficient erythropoiesis and poor iron status, compared to normal weight individuals. Because iron is required for the activity of tyrosine hydroxylase, it might be that the obese have reduced activity of this enzyme through diminished iron availability. This would result in a decrease in motivation, depressed mood and decreased physical performance. Therefore reductions in tyrosine hydroxylase could explain the association between exercise and body weight (here). In fact it is known that catecholamine release is diminished in the obese following ingestion of a meal (here), suggesting that tyrosine hydroxylase activity is impaired. Because catecholamines are required for lipolysis, decreased tyrosine hydroxylase activity could also explain some of the difficulties in causing weight loss in those with metabolic syndrome.

