Dietary protein must be broken down to amino acids and shorter peptides to facilitate absorption in the gut. This process is achieved by the release of proteases into the lumen of the gut, and is aided by the acidic environment of the stomach. The proteases and low pH cause hydrolysis of the peptide bonds in the protein and this produces an array of different sized exogenous derived peptides, which are subsequently absorbed via active transport processes. However, exogenous protein is accompanied by secretion of an equal quantity of endogenous proteins that becomes hydrolysed. This combination of exogenous and endogenous proteins causes the formation of an extremely complex protein mixture. The interaction of this protein mixture with receptors in the gut is not fully understood, but derivatives of endogenous proteins are thought to elicit physiological changes to gut physiology. In particular these protein derived peptides may possess opioid and antihypertensive effects and as such may have important implications to human health.
To understand the digestion of protein and the production of various bioactive peptides, researchers have used nasogastric tubes to sample the products of protein digestion in humans. For example, in one study1 subjects ingested 30 grams of casein or whey protein, and researchers measured the nitrogen flow rates and the production of peptides from gut samples. The results showed that the kinetics of whey and casein digestion differed considerably. For casein, nitrogen delivery from the stomach to the small intestine continued for 6 hours, whereas the delivery of nitrogen for whey protein was more rapid, being completed in only 3 hours (figure 1). This supports previous studies of these two proteins that show whey is quickly absorbed, whereas casein has a more controlled and delayed absorption profile. However, the secretion of endogenous protein (such as pancreatic juice) to the small intestine was not different between the two milk proteins. After 6 hours total dietary nitrogen absorption from the jejunum was 50 % for casein and 60 % for whey protein.
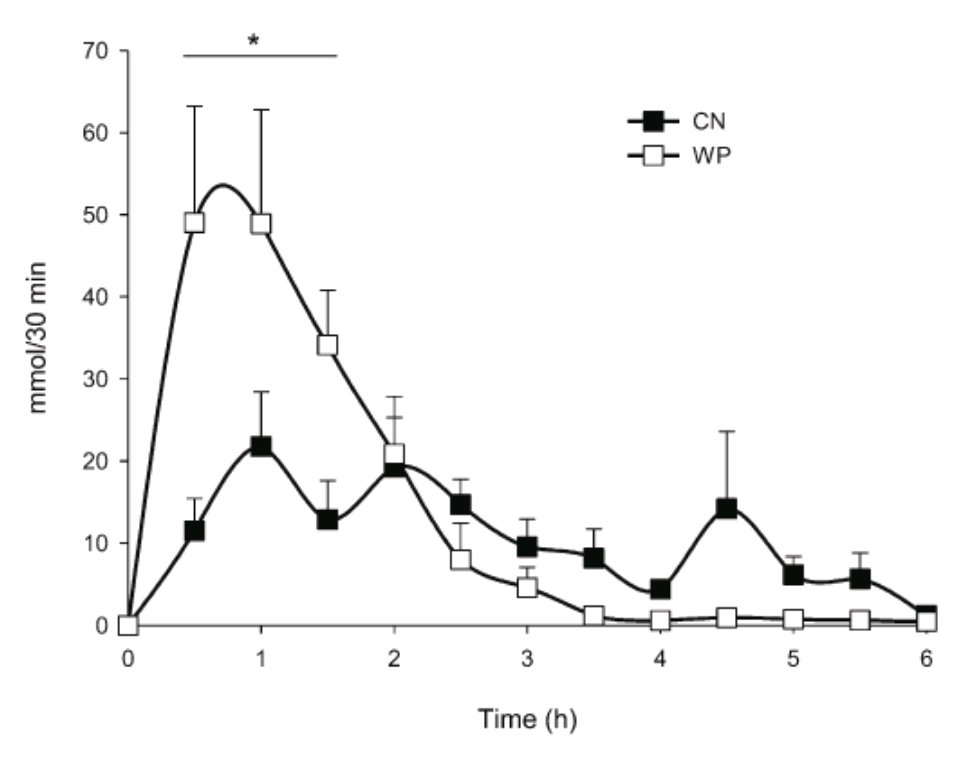
Figure 1. The absorption kinetics of nitrogen delivery to the small intestine for whey protein and casein. Whey protein produces a large influx of nitrogen to the small intestine rapidly within 3 hours, whereas casein produces a more prolonged delivery over 6 hours.
The peptides detected in the small intestine differed between whey and casein treatments. Following casein ingestion, medium sized peptides of between 750 and 1050 kDa were detected during the 6 hours transit time to the distal small intestine. However, for whey protein the shorter 3 hour delivery from the stomach to the distal small intestine resulted in larger 1050 to 1800 kDa peptides. In total 356 different peptides were detected after ingestion of the casein protein, compared to only 146 with the whey protein. Of the proteins present in milk (αS1-casein, αS2-casein, β-casein and κ-casein), β-casein was the most important precursor of bioactive peptides with 218 derivatives (61.2 %), whereas αS1-casein, αS2-casein and κ-casein represented 24.9 , 8.2 and 6.7 %, respectively. Derivatives of β-casein that are known to illicit opioid and antihypertensive effects (β-casomorphin and β-casein 108-113 kDa) were present in high enough concentrations in the postprandial window in order to cause significant physiological effects.
These results suggest that casein ingestion causes a more prolonged absorption of nitrogen in comparison to whey protein. The fact that casein was able to cause a prolonged production of a wide variety of medium sized peptide, in contrast to the rapid 3 hour release of fewer but longer peptides, suggests that these two proteins have quite different physiological effects. In addition, casein causes the production of bioactive peptides that are not produced from whey protein. For example, no known bioactive peptides of the main proteins in whey (including α-lactoglobulin, β-lactoglobulin, lactoferrin and bovine serum albumin) were detected. This suggests a quite different biological activity between whey and casein proteins and highlights the complexity of protein digestion beyond the simple nitrogen ratings often given to them. The opioid and antihypertensive effects of casein derived peptides suggests that casein could have nutritional functions other than as a source of protein, but this requires further research.
RdB
