 Folic acid is chemically named N-[4-(2-amino-4-hydroxy pteroyl-(6-methylamino)-benzoyl]- L-glutaric acid or pteroylglutamic acid (PGA). Folic acid belongs to the B vitamin group of vitamins and is sometimes called vitamin B9, vitamin B11, vitamin M or folacin. The chemical structure of folic acid is given in figure 1. The structure consists of a pterin ring linked to para aminobenzoic acid (PABA), which in turn is linked to a single molecule of glutamic acid. Folates are generic terms that relate to compounds that have the same activity and folic acid, but which differ in structure. Whereas folic acid is a synthetic compound, other folates are found in nature. Natural folates are found mainly in plants, but also in animals that have eaten and then stored folates from plants. There are currently over 20 known folates in nature that have been characterised and these are referred to as pteroylpolyglutamates. The main natural folates found in food include N5 methyltetrahydrofolate (THF) and N10 formyltetrahydrofolate.
Folic acid is chemically named N-[4-(2-amino-4-hydroxy pteroyl-(6-methylamino)-benzoyl]- L-glutaric acid or pteroylglutamic acid (PGA). Folic acid belongs to the B vitamin group of vitamins and is sometimes called vitamin B9, vitamin B11, vitamin M or folacin. The chemical structure of folic acid is given in figure 1. The structure consists of a pterin ring linked to para aminobenzoic acid (PABA), which in turn is linked to a single molecule of glutamic acid. Folates are generic terms that relate to compounds that have the same activity and folic acid, but which differ in structure. Whereas folic acid is a synthetic compound, other folates are found in nature. Natural folates are found mainly in plants, but also in animals that have eaten and then stored folates from plants. There are currently over 20 known folates in nature that have been characterised and these are referred to as pteroylpolyglutamates. The main natural folates found in food include N5 methyltetrahydrofolate (THF) and N10 formyltetrahydrofolate.
Animals cannot synthesize folate because they cannot synthesise PABA nor bond glutamate to it. Plants, particularly green leafy vegetables, or yeast, must therefore provide animals with folates. Natural folates differ from folic acid in two main ways. Firstly, natural folates have more that one glutamate residue linked to the PABA moiety. This is why natural folates from food are referred to a pteroylpolyglutamates, a reflection of the many glutamate residues they possess. In contrast, folic acid has one glutamate and is referred to a pteroylmonoglutamate. Secondly, natural folates may have carbon substituents on the N5 and N10 moieties of the molecule, with a methyl group bonded to the N5 group producing N5 methyltetrahydrofolate and a formyl group bonded to the N10 group producing N10 formyltetrahydrofolate. Folates are degraded by light and heat and this can cause cleavage, and therefore destruction of the molecule. They have limited solubility in water, but is soluble in acidic conditions as may be found in the stomach.
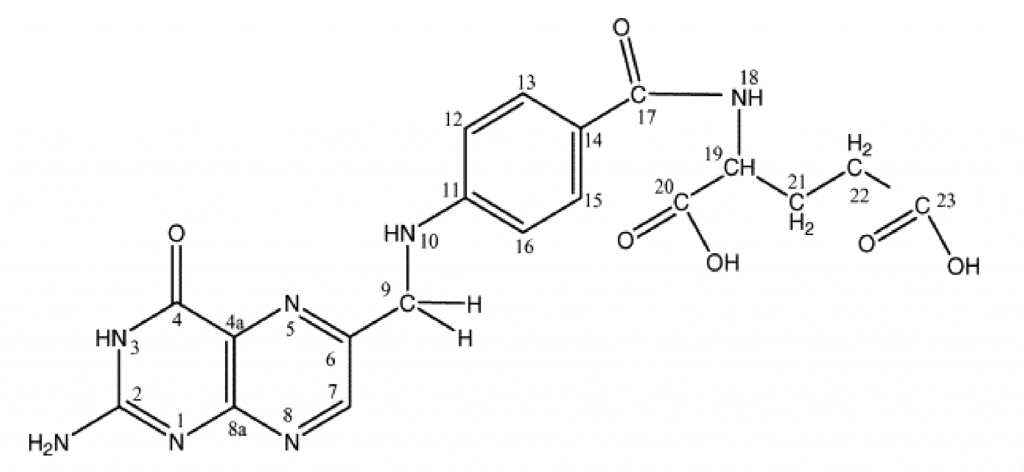
Figure 1. The structure of folic acid (see: Tuszyńska, 2012). There are two main differences between synthetic folic acid (pictured) and other natural folates. Fairly, natural folates have multiple glutamate residues, whereas folic acid has a single glutamate residue. This is why folic acid is called pteroylmonoglutamate and other natural folates are called pteroylpolyglutamates. Secondly, natural folates can have a one carbon substituent group on the N5 and N10 positions of the molecule. For example, a methyl group on the N5 group produced N5 methyltetrahydrofolate, and a formyl group on the N10 position produces N10 formyltetrahydrofolate. N5 tetrahydrofolate and N10 formyltetrahydrofolate are the two most common folates in human nutrition. In the blood folates are present as conjugated tetrahydrofolate monoglutamate. These are taken up by cells, and they are then converted to polyglutamates, where they can then take part in carbon (methyl) transfer reactions. One carbon group conjugated tetrahydrofolate polyglutamates are therefore the biologically active forms of folates in humans and other animals. When humans eat animal tissue, they consume these active forms of folate from the animal cells.
Folates are absorbed as monoglutamates. Therefore natural folates with multiple glutamate residues undergo a cleavage reaction that removes all but one glutamate residue. The enzyme that performs this is called γ-glutamyl carboxypeptidase, an enzyme that is present in the brush border of the intestine. Absorption of folates occurs mainly through the jejunum of the small intestine. Within the intestine, folate is typically reduced to tetrahydrofolate and then methylated or formylated to N5 methyl tetrahydrofolate or N10 formyl tetrahydrofolate, respectively. The folate is then transported to the liver through the hepatic portal circulation, mainly in the N5 methyltetrahydrofolate, with lower amounts of N10 formyltetrahydrofolate and some dihydrofolate. The N5 methyltetrahydrofolate and N10 formyltetrahydrofolate enter the bile, and are they reabsorbed in the enterohepatic circulation to enter the blood. The dihydrofolate is reduced to tetrahydrofolate, conjugated and released to circulation.
Eat Well, Stay Healthy, Protect Yourself
RdB
