Glycolysis is perhaps the most important pathway in biochemistry because it is central to so many other pathways and is considered important in energy metabolism in almost all living organisms. The third step in the glycolytic pathway involves the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, a reaction catalysed by the enzyme phosphofructokinase-1 (see figure 1). This enzyme is an important regulatory step in glycolysis, and the enzyme is so named to distinguish it from phosphofructokinase-2, which produces fructose 2,6-bisphosphate, a potent allosteric stimulator of phosphofructokinase-1. Because fructose 2,6-bisphosphate stimulates phosphofructokinase-1 it has an important influence on the rate of glycolysis. In addition, in liver tissue fructose 2,6-bisphosphate inhibits the gluconeogenic enzyme fructose 1,6-bisphosphatase, thereby inhibiting the conversion of glucogenic amino acids into glucose via the gluconeogenic pathway. In liver, the concentration of fructose 2,6-bisphosphate is regulated by insulin and glucagon.
Glucagon release stimulates the production of cyclic AMP (cAMP). As cellular levels of cAMP rise, the active catalytic monomers of cAMP dependent protein kinase become free to phosphorylate the bifunctional enzyme phosphofructokinase-2 / fructose 2,6-bisphosphatase. When phosphofructokinase-2 / fructose 2,6-bisphosphatase is phosphorylated in liver, phosphofructokinase-2 is inactive and fructose 2,6-bisphosphatase is active. This decreases the concentration of fructose 2,6-bisphosphate because it is converted to fructose 6-phosphate by fructose 2,6-bisphosphatase. Low levels of fructose 2,6-bisphosphate cause the inhibition on fructose 1,6-bisphosphatase to be released this increasing gluconeogenesis. When insulin is released, cAMP levels fall and the bifunctional enzyme becomes dephosphorylated which activates phosphofructokinase-2 and inactivates fructose 2,6-bisphosphatase. This increases levels of fructose 2,6-bisphosphate which then stimulates phosphofructokinase-1 and glycolysis (figure 2). In muscle, high cAMP (caused by adrenaline release) leads to phosphofructokinase-2 that is active and fructose 2,6-bisphosphatase that is inactive thereby stimulating glycolysis.
 Figure 1. Diagram showing glycolysis and gluconeogenesis.
Figure 1. Diagram showing glycolysis and gluconeogenesis.
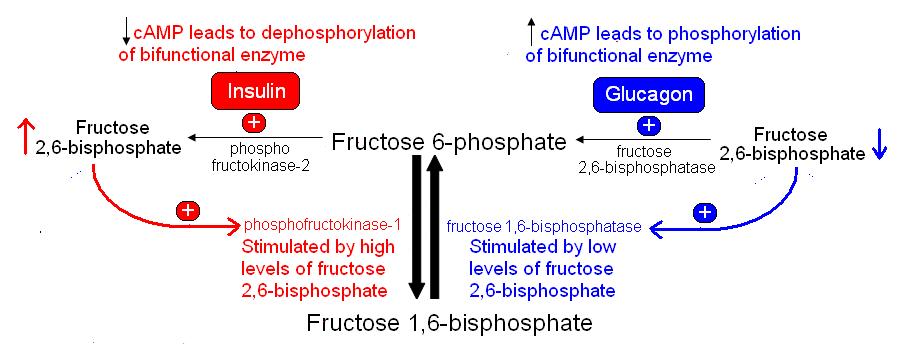 Figure 2. Allosteric control of glycolysis and gluconeogenesis by fructose 2,6-bisphosphate.
Figure 2. Allosteric control of glycolysis and gluconeogenesis by fructose 2,6-bisphosphate.
RdB
