Flavonoids belong to a group of natural compounds with variable low molecular weight polyphenolic structures found in material of plant origin. Their structure is based on a flavan nucleus which consists of three phenolic rings referred to as the A, B and C rings (figure 1). The benzene ring A is condensed with the six member C ring, which in the 2-position carries the B ring as a substitute. Variations in the heterocyclic C ring give rise to the main groups of flavonoids (figure 2).
Figure 1. The flavan nucleus common to all flavonoids, consisting of the A, B and C rings.
Structurally, there are two main groups of flavonoids, the 2-phenylchromans (flavonols, flavanones, flavan-3-ols, flavones, anthocyanins and the condensed tannins (proanthocyanidins)) and the 3-phenylchromans (the isoflavonoids, including isoflavones, isoflavans and pterocarpans). The isoflavonoids are a specialised form of flavonoids that are synthesised by certain legume plants (such as the soybean: Glycine max) and a small number of non-legume plants. The basic structure of flavonoids allows a large number of substitutions in the three rings; variable numbers of hydroxyls may be further substituted with sugars, methyl groups, sulphates and glucuronides, thus producing an extremely diverse range of derivatives.
The chalcones are aromatic ketones and although closely related, are not true flavonoids. The lack the aromatic C ring in their structure. The most common dietary chalcones are phloretin, phloretin 2’-O-glycoside (phloridzin), chalconaringenin and arbutin. Apples are a common food source of phloretin and phloridzin. Similarly, a closely related group of chemicals called the stilbenes are synthesised by grape (Vitis vinifera), peanut (Arachis hypogaea) and pine (Pinus sylvestris). The stilbene trans-resveratrol has been extensively researched with regard its possible protective role in cardiovascular disease. Other stilbenes include trans-piceid, pinosylvin, piceatannol, pinosylvin, trans-pterostilbene, astringin and rhapontin.
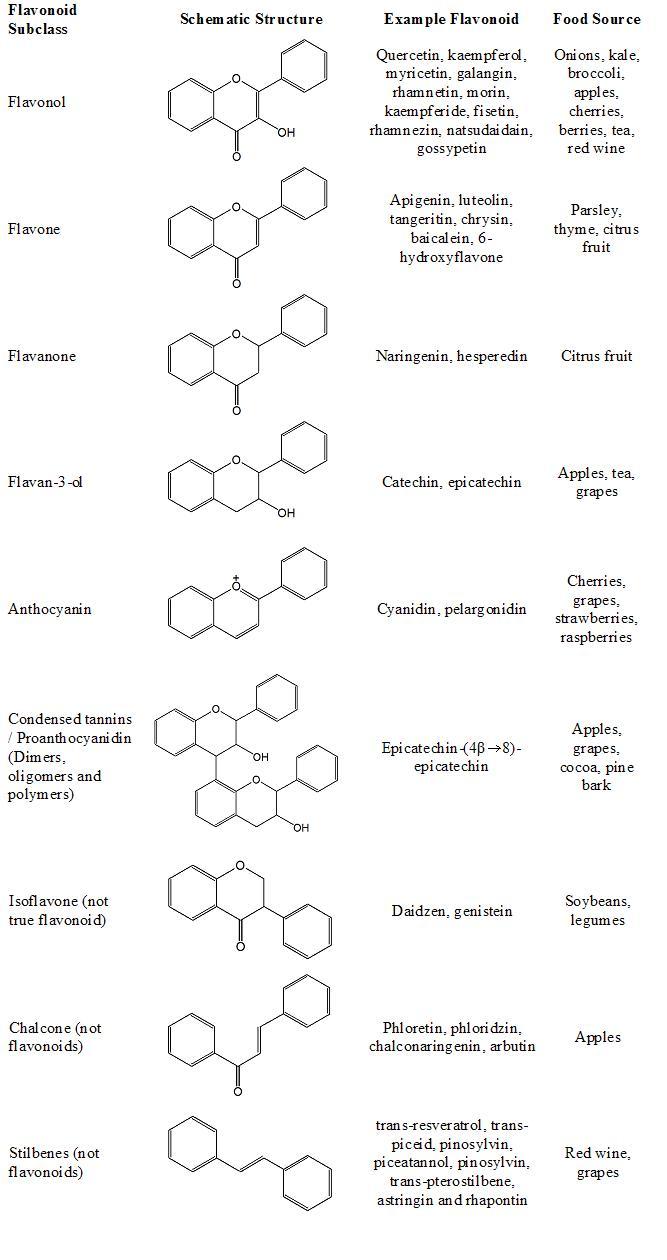 Figure 2. The structure of flavonoids including the non-flavonoid groups, the stilbenes and chalcones.
Figure 2. The structure of flavonoids including the non-flavonoid groups, the stilbenes and chalcones.
RdB

