Electrons circle the nucleus of atoms and are usually present in shells as pairs. Free radicals are atoms, usually in molecules (excluding metals), that have electrons that are no longer paired. Free radicals that contain oxygen are referred to as reactive oxygen species. Figure 1 shows a list of the most common free radicals found in biological systems. Free radicals are formed during normal metabolisms in the body, but the amount of free radicals can be increased dramatically though exposure to pollution, various chemicals, drugs and radiation. Free radicals are reactive because of the unpaired electrons, and this can lead to cellular damage and disease. Free radicals often propagate chain reactions whereby one radical reacts with cellular components, which then itself becomes a radical, only to react with further cellular components in a chain reaction.
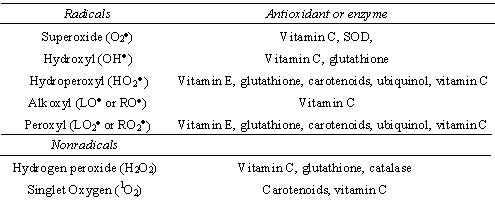 Figure 1. Examples of some reactive oxygen that are free radicals (Right). R = non lipid molecule, L = lipid molecule. Some tissue damaging nonradicals are also included. The antioxidants able to quench the radicals and nonradicals are also shown (left).
Figure 1. Examples of some reactive oxygen that are free radicals (Right). R = non lipid molecule, L = lipid molecule. Some tissue damaging nonradicals are also included. The antioxidants able to quench the radicals and nonradicals are also shown (left).
Antioxidants are chemical compounds that are able to quench free radical and stop the propagation of the damaging chain reactions. Generally antioxidants are reducing agents, in that they are able to donate electrons to free radicals to stabilise their structure. The antioxidant is oxidised in this process and so becomes a free radical itself. However, generally antioxidants do not cause tissue damage once oxidised for two main reasons. Firstly, some antioxidants have structures that are able to delocalise the charge of the unpaired electron around the molecule, thus stabilising the structure. Flavonoids and vitamin E are able to do this. Secondly, the body has an intricate system that allows it to recycle oxidised antioxidants back to their reduced state (figure 2). The reduced antioxidants are then able to further reduce other free radicals.
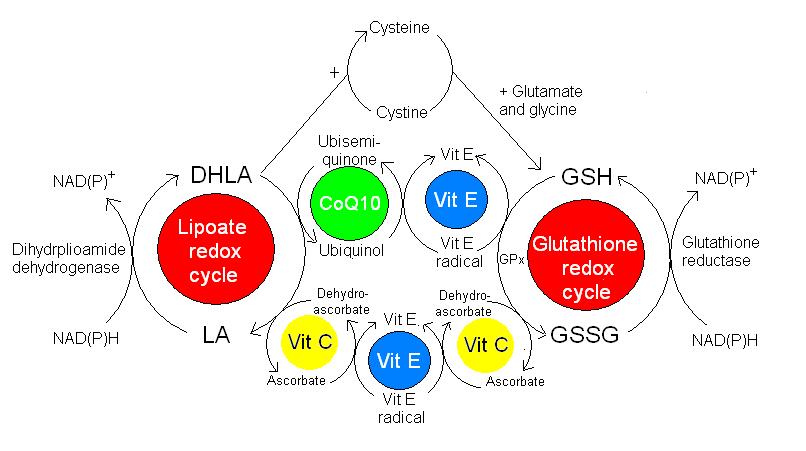 Figure 2. A schematic diagram to demonstrate the recycling of various antioxidants.
Figure 2. A schematic diagram to demonstrate the recycling of various antioxidants.
Some antioxidants are produced naturally in our bodies. For example, glutathione and lipoic acid are thiol reducing agents produced in human cells that play an important role in the antioxidant defence against free radicals. However, many of the antioxidants active in humans are derived from the foods that we consume (e.g. carotenoids and flavonoids). Evidence is starting to mount that increasing the amount of antioxidants that we consume is protective of a number of diseases including cancer and heart disease. In fact, many of the foods traditionally used in ancient forms of medicine to treat common diseases are being discovered to be rich sources of chemicals which are good reducing agents. Knowing which foods contain high levels of these chemicals is therefore important because it allow the incorporation of these foods into our diets.
A study published in the American Journal of Clinical Nutrition in 20061, investigated the antioxidant contents of various foods. Researchers used an assay that measures the total concentration of antioxidants to analyze 1113 food samples. Using the data they obtained the researchers complied a table of the top foods with the best antioxidant ability per serving size (figure 3). Many of these top antioxidant foods are berries which contain high levels of anthocyanins, a flavonoid compound (here). The researchers also noted that large variation in the antioxidant content of many foods was detected. The richest source of antioxidants were herbs and spice, nuts and seeds, berries and fruit and vegetables. Intakes of many of these foods are inversely associate with degenerative disease, and so incorporating a number of these foods into the diet is an important part of optimum nutrition.
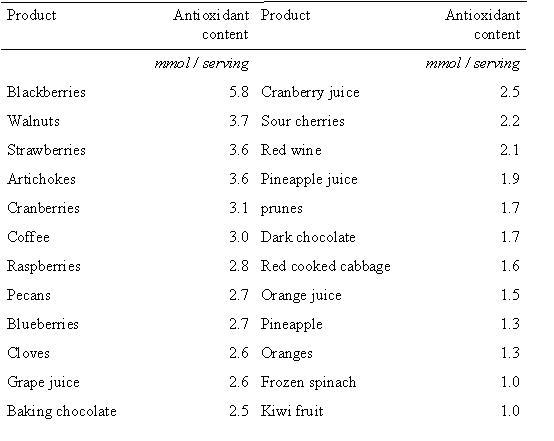 Figure 2. A selection of foods that are rich in total antioxidant conent1.
Figure 2. A selection of foods that are rich in total antioxidant conent1.
RdB
