Free radicals are chemicals that possess an unpaired electron in their outer orbital. This chemical structure makes them reactive and interaction with other chemicals can cause the free radical to gain an electron. Such interactions reduce and stabilise the free radical (which now has a full compliment of electrons), but at the same time oxidises and destabilises the other chemical creating a new free radical (which is now missing an electron). Free radicals are a normal and necessary part of cellular metabolism because electron transfer is the basis for energy production in cells. Under healthy conditions the cell can limit the damaged caused by free radicals because it has antioxidant defences. Antioxidants are powerful reducing agents that can donate electrons to free radicals, thus inhibiting the dangerous chain reactions before they have a chance to damage cellular components. The main antioxidant defences in cells are made up of endogenously synthesised enzymes along with dietary vitamins.
Cellular antioxidants in animals cells form a complex synergistic network of protection from free radicals. Free radicals produced from energy metabolism or from endogenous chemicals often react with fatty acids in the membranes of cells resulting in the production of lipid peroxides. Vitamin E is the primary defence against lipid peroxidation and donates an electron to the lipid peroxide, stabilising its structure. In the process vitamin E becomes the tocopherol radical. This tocopherol radical then reacts with vitamin C forming vitamin E and dehydroascorbate, the oxidised form of vitamin C. Dehydroascorbate then reacts with reduced glutathione in a reaction catalysed by the selenium requiring enzyme glutathione peroxidase. This forms oxidised glutathione, which is returned to its reduced state in a reaction involving NADPH and glutathione reductase. The NADPH is produced in the pentose phosphate pathway and the glutathione reductase is a vitamin B2 (riboflavin) requirement enzyme (figure 1).
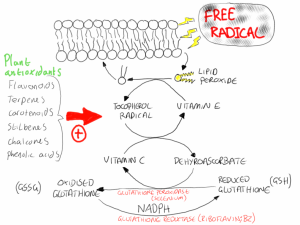
Figure 1. The synergism between antioxidants produces a complex protective system that limits the damage that can be caused to cellular components by free radicals.
This antioxidant merry-go-round is interesting nutritionally because it requires dietary vitamin E, vitamin C, selenium and riboflavin to function. Deficiencies in any of these dietary nutrients impairs the antioxidant defences of cells and causes tissue damage and disease. Tissues exposed to high amounts of free radicals such as the eye require much higher concentrations of these antioxidants than other tissues. Red itchy eyes for example are a symptom of riboflavin deficiency, and this results from a breakdown of the glutathione reductase enzyme system, such that oxidised glutathione builds up in the conjunctiva of the eye causing free radical damage that induces inflammation, thereby leading to redness and itching. Glutathione therefore may be integral to the protection of the eye. In fact it is known that levels of glutathione reductase are reduced in cases of cataract formation, which has lead to speculation that riboflavin deficiency may be a causative factor in the formation of cataracts.
However, studies that have looked at the role of riboflavin in cataract formation have not always found clear evidence of involvement of riboflavin deficiency. For example, in one study1, researchers assessed the riboflavin status of healthy young adults, older cataract patients and older subjects with clear lenses. There was no evidence of an association between early cataract formation and riboflavin deficiency. However, the authors did report that older cataract patients were more likely to have riboflavin deficiencies than older patients with clear lenses. This association of course does not prove cause and effect, but is interesting nonetheless. In addition, it is difficult to conclude that a single antioxidant component is at fault in a complex interactive system. For this reason more recent research has centred more on antioxidant defences as a whole rather than individual components. Results from such studies have confirmed the role of antioxidant defences in disease prevention, including that of the eye.
Dr Robert Barrington’s Nutritional Recommendation: Antioxidants are important components of the diet., However, it is naive to focus solely on the major vitamins. Both vitamin C and vitamin E are undoubtedly important in their role as antioxidants. However, plants provide a wide range of antioxidants including carotenoids, flavonoids, chalcones, stilbenes, terpenes, phenolic acids and other chemicals that may be required for optimal antioxidant defences. The best way to fortify these antioxidant defenses to protect vital tissues such as the eye, is to eat a wide range of plant foods made up of a range of different colours. For example, the purple, blue and red colours in berries are a reflection of the flavonoids they contain, whereas the yellow, red and orange colours of tomatoes and peppers reflects the carotenoids they contain. Supplementing with a range of vitamin E isomers is also important as the individual isomers may have slightly different biochemical roles in the antioxidant defence systems.
RdB
