 Anthocyanins are a group of phytonutrients with polyphenolic structures. They belong to the flavonoid group of nutrients and possess A, B and C rings with various subgroups that produces a range of different chemicals. Anthocyanins are found in plants as anthocyanidins, which are chemically anthocyanins with sugar groups attached. The large number of possible combinations of functional groups, with a variety of sugars produces a range of anthocyanidins that include malvidin, cyanidin, peonidin, petunidin, rosinidin, delphinidin and europinidin. The general chemical structure of an anthocyanin is given in figure 1. Anthocyanins and their glycosides show a range of colours in nature, and these colours are generally red, blue and purple. Anthocyanins are responsible for many of the red, blue and purple colour seen in fruits, petals and vegetables for example. The colour of anthocyanins can be changed through chemical means and this allows the same anthocyanins to show a number of different colours in foods.
Anthocyanins are a group of phytonutrients with polyphenolic structures. They belong to the flavonoid group of nutrients and possess A, B and C rings with various subgroups that produces a range of different chemicals. Anthocyanins are found in plants as anthocyanidins, which are chemically anthocyanins with sugar groups attached. The large number of possible combinations of functional groups, with a variety of sugars produces a range of anthocyanidins that include malvidin, cyanidin, peonidin, petunidin, rosinidin, delphinidin and europinidin. The general chemical structure of an anthocyanin is given in figure 1. Anthocyanins and their glycosides show a range of colours in nature, and these colours are generally red, blue and purple. Anthocyanins are responsible for many of the red, blue and purple colour seen in fruits, petals and vegetables for example. The colour of anthocyanins can be changed through chemical means and this allows the same anthocyanins to show a number of different colours in foods.
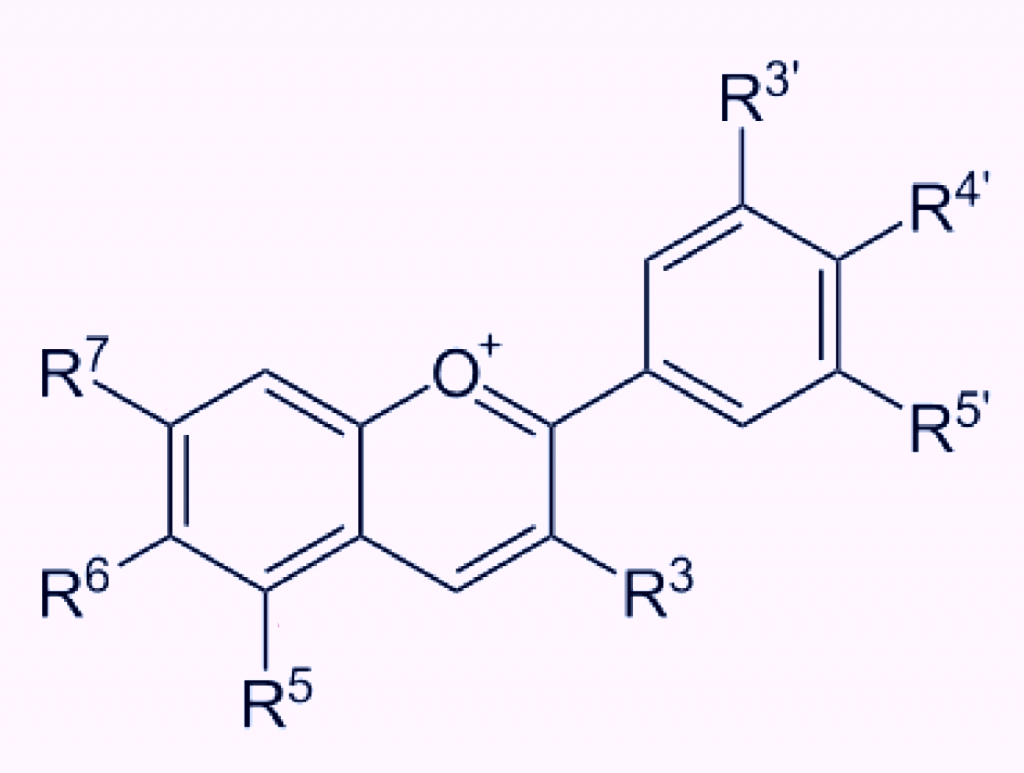
Figure 1. The general chemical structure of an anthocyanin. The R groups (also called variable groups) can vary in the functional groups they bond, but are limited to manly -OH (hydroxyl), -H (hydrogen) or -OCH3 (methyl) groups. Combinations of these groups on the R groups produces a range of anthocyanins. Anthocyanidins are produced when a sugar such as glucose bonds to the anthocyanin molecule. This bonding is usually at the R3 position. A range of possible sugars can produce a range of anthocyanin glycosides (anthocyanidins).
For example, anthocyanins are known to be able to be used as pH indicators. Beetroot and red cabbage can be homogenised in a blender and then added to solutions of various pH in order to determine the hydrogen ion concentration of the solutions. Acid solutions with a relatively high concentration of hydrogen ions in solution with turn red when this homogenised mixture is added. In contrast, alkaline solutions that contain relatively few hydrogen ions in solution will turn blue and then yellow as the hydrogen ion concentration drops. In water of neutral pH, the anthocyanins remain purple. This occurs because the hydrogen ions interact with the anthocyanins changing their colour, and this demonstrates the range of colours that can be produced from the same anthocyanins, when exposed to different chemical environments. Cabernet Sauvignon wines have a pH range from ~3.0 to 3.9 and therefore the anthocyanins in the wine solution differ in colour and change the appearance of the wine depending on the pH.

Anthocyanins in blueberries skins are blue. This results from complexation of metal ions with the B ring of the anthocyanins. Because such complexation can only occur with those anthocyanins containing two hydroxyls on the B ring, some basic prediction of the anthocyanins present in blueberries is possible. Cyanidin, delphinidin and petunidin all contain two hydroxyls on their B ring, and so it is likely that blueberries contain one or all of these anthocyanins. In fact blueberries contain cyanidin, delphinidin and petunidin. However, they also contain malvidin and peonidin. Because of the chemical complexity of anthocyanin chemistry and the fact that different anthocyanins show different health effects, eating a range of plants containing red, blue and purple colours should ensure the widest possible range of anthocyanins is present in the diet, and this may confer the best health effects.
A number of metal ions are known to interact with anthocyanins in nature and change the appearance of plant foods. Aluminium ions for example interact with the B ring of anthocyanins and change the colour from red to blue. This process is responsible for the blue colour of blueberry skins. Other metal ions that are known to influence the colour of anthocyanins include iron, selenium, boron and copper ions. However, such interactions are limited to the ortho-dihydroxy arrangement on the B ring, and so while such complexes are possible with glycosides of cyanidin, delphinidin and petunidin (because they contain two hydroxyl groups on the B ring), they are not possible with malvidin, pelargonidin and peonidin (because they contain only 1 hydroxyl group on the B ring). It is therefore difficult to predict the colour that anthocyanins will show in nature, and this makes their identification visually difficult. Predicting the type of anthocyanins present in foods based on colour is therefore not possible.
Eat Well, Stay Healthy, Protect Yourself
RdB
